SERS-based Ultrasensitive Identification and Quantification of Drugs in Biofluids
The enormous increase of Raman signal in the vicinity of metal nanoparticles allows surface-enhanced Raman spectroscopy (SERS) to be employed for label-free detection of substances at extremely low concentrations. However,
the ultimate potential of label-free SERS to identify pharmaceutical compounds at low concentrations, especially in relation to biofluid sensing, is far from being fully realized. Opioids are a particular challenge
for rapid clinical identification because their molecular structural similarities prevent their differentiation with immunolabeling approaches. Recently (Small 2018)
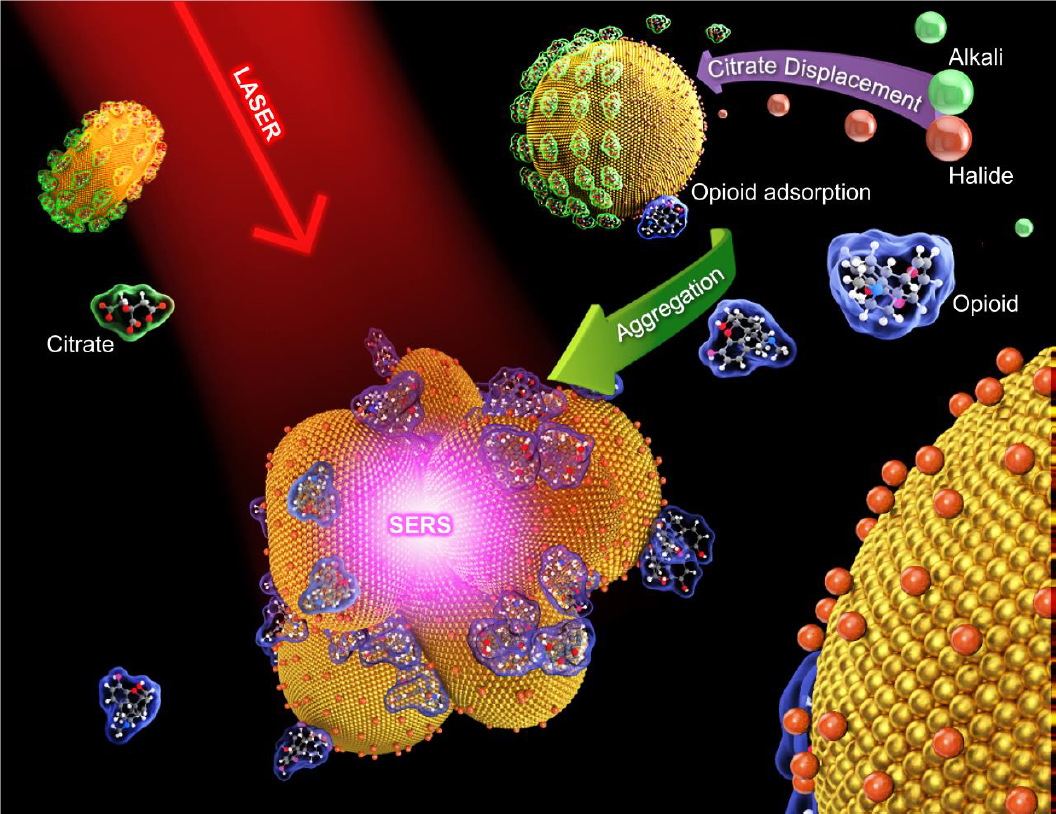
we developed a new method called quantitative label-free SERS (QLF-SERS) which involves the formation of halide-conjugated gold nanoclusters trapping the analyte of interest near the SERS hot spots. QLF-SERS shows a
dramatic improvement in sensitivity over existing label-free SERS methods. We also demonstrate the QLF-SERS algorithm which achieves rapid quantitative drug identification in clinical urine samples containing multiple
components at concentrations that rival currently existing laboratory urine drug testing techniques but is significantly faster and inexpensive and, therefore, could be easily adapted as part of a rapid clinical laboratory
routine.
Our results indicate that nanoclusters that produce the highest SERS signal are small aggregates which trap the analyte of interest near the SERS hot spots. Larger aggregates become less efficient at producing the SERS
signal because sites which would normally result in SERS hot spots become shielded by the outer layers of the nanostructure. Importantly, generating the SERS signal also requires the chemical to be in the vicinity of
the hot spot and therefore, making the ideal structure a composite of nanoparticles and analyte molecules. The self-assembly of this structure is determined by 1) the affinity of the analyte molecules to the nanoparticles,
and 2) the relative diffusion rates of the analyte molecules and the nanoparticles. These factors can be controlled by the type and amount of aggregation agent, the size and concentration of the nanoparticles, and the
sequence of steps in nanocluster preparation. The aggregating agent and its concentration affects both the affinity of the analyte to the nanoparticle and the aggregation rate of the nanoparticles.
Our work demonstrates
that label-free SERS measurements can be far more sensitive than previously thought, achieving detection limits of 5 pg/mL. This detection limit is 1,000 times lower than any previously reported label-free SERS measurement
of a pharmaceutical compound and 100,000 times lower than any previously reported label-free SERS measurement of opioids. This dramatic improvement is largely achieved by optimizing the aggregation conditions, thus
optimizing the size and surface chemistry of the nanocluster. The detectable concentrations achieved in our study are only an order of magnitude higher than those achieved for single molecule detection.